Participating in research
– Overview
– How is research different from treatment?
– Who can participate in research?
– What are the different types of research?
– How do researchers collect data?
– How do researchers use the data they collect?
– It's your decision to participate
– Asking questions
Overview
The University of Minnesota is a leading public research institution. A wide variety of research studies, from behavioral studies to experimental drug studies, take place here. By volunteering for research, you can help researchers discover answers to questions to improve people’s lives.
Learn more about research at the University of Minnesota
How is research different from treatment?
- The goal of research is to learn new things in order to help groups of people in the future. Researchers learn things by following the same plan with a number of participants, so they do not usually make changes to the plan for individual research participants. You, as an individual, may or may not be helped by volunteering for a research study.
- The goal of treatment is to help you get better or to improve your quality of life. Doctors can make changes to your treatment plan as needed.
Who can participate in research?
Researchers look for people who fit a certain description, called eligibility criteria. These criteria give details on who can and cannot join a study and could include:
- People of a certain age or gender
- People who do or do not have a certain illness, disease, or health condition
- People with or without a certain health history, such as a prior treatment
- People who are exposed to or are in contact with something that affects their health
Researchers use eligibility criteria to keep participants safe and enroll the right participants to collect the data they need to answer the research question. There are many kinds of research studies, all with different types of eligibility criteria.
What are the different types of research?
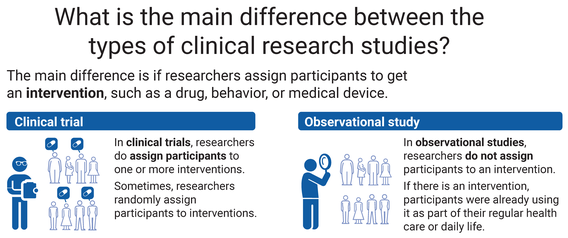
Both may try to learn more about an intervention, which may be a drug, behavior, or medical device. The main difference is clinical trial participants are assigned to get an intervention, but observational study participants are not assigned to get an intervention.
Clinical trials
Clinical trials are research studies in which researchers assign participants to get one or more interventions to test what happens in people. Because of this, clinical trials are also called interventional studies. Often, the intervention is investigational, which means it is not approved for doctors to prescribe to people.
In some clinical trials, researchers assign participants to interventions randomly. This means that researchers assign the participants by chance. Usually, participants (or their doctors) don't choose what intervention they will get when they join a clinical trial.
Observational studies
Observational studies are research studies in which researchers simply collect information (called data) from participants or look at data that was already collected. The data may be about participants’ health, habits, or environments. In observational studies, researchers do not assign participants to get an intervention. If there is an intervention, participants were already using it as part of their regular health care or daily life.
Often, researchers use observational studies to look at (observe) the different ways people behave and how it affects their health. Some observational studies use patient registries. A patient registry is an organized collection of data that patients agree to give. Researchers can use a patient registry to quickly access data provided by hundreds, or thousands, of similar patients.
Compare the 2 types of clinical research
How do researchers collect data?
During the study, researchers collect data from participants to help answer their research question. They do this in different ways, such as:
- Surveys or questionnaires
- Getting images, such as X-rays or MRIs
- Taking measurements, such as height, weight, or blood pressure
- Taking samples of participants’ blood or tissue to look at in a lab
Researchers may need to collect data from participants many times or only a few times.
How do researchers use the data they collect?
Researchers analyze (study) the data they collect from participants based on the research plan to answer their research questions. The informed consent form describes what researchers plan to do with your data.
After the researchers analyze the data and the study is complete, researchers can share the study results. Study results summarize group data collected from all participants. These results can be published in research journals. In some cases, researchers may list data from individual participants, but not in a way that allows readers to identify the participant.
It's your decision to participate
When thinking about volunteering for a research study, you can:
- Talk with family, friends, or others before making a decision
- Ask as many questions as you want
- Change your mind at any time
- Leave a research study knowing that the treatments or services that you receive from your doctor, clinic, hospital, or others will not be affected
Asking questions
Before volunteering for a research study, you should know the answers to these questions:
- What is the research study about?
- How is this different from my treatment plan?
- What will I be asked to do?
- How much time will it take?
- Are there potential side effects or risks?
- What will happen if I have problems because of participating?
- Are there treatment options I should know about? • How will my information be kept private or shared?
- Will I be billed for any costs?
- How will I be compensated for my time?
- Who can I contact with questions or concerns?
- How do I leave the study after I start?